|
|
Tissue
Mechanics Laboratory |
 |
Research
Interests
Our research
mainly spans from experimental and computational biomechanics to
mechanical behaviour of biological tissue, particularly in understanding
the relationship between tissue hierarchical structures at multiple
length scales (ranging from organ down to cellular level) and their
physiological as well as pathological conditions. Our research is highly
interdisciplinary and at the interface of engineering, materials science
and biomedicine.
Research Highlights
| Tissue Mechanics - From
Multiscale Structures to Digital Diagnostics |
 |
| Funded by EPSRC |
|
-
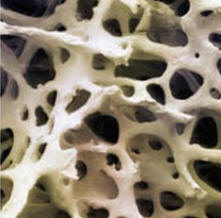
In collaboration with Prof. Ke Chen (Liverpool), Dr. Pankaj
Pankaj (Edinburgh) and Dr. Junjie Wu (Durham)
Biological tissue is a highly heterogeneous, anisotropic
material with structural features at multiple length scales.
Tissue functions and behaviours in both healthy and diseased
models often largely rely on its microstructure. This project
aims to evaluate the tissue quality using microstructural
analysis, nonlinear mechanics and homogenisation method and
design a computational tool using new microstructural indices
for early diagnosis of microstructure-related disease at tissue
level.
|
| Prostate
Tissue Mechanics - Microstructural
Analaysis for Cancer Diagnostics |
 |
| Funded by EPSRC |
|
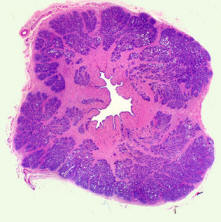
-
In collaboration with Prof. Bob Reuben (Heriot-Watt) and
Edinburgh Western General Hospital
Prostate cancer is usually preliminarily diagnosed by Digital
Rectal Examination (DRE) due to the fact that cancerous
prostatic tissue is often mechanically stiffer. This project
aims at modelling the mechanical behaviour of prostate tissue
(highly porous, interconnected and viscoelastic but may change
subject to various pathological conditions) under different ways
of mechanical palpation therefore identifying the most effective
means to enable minimally-invasive early diagnostics of prostate
cancer using 'probes-on-a-finger' device.
|
| In Vivo Tissue
Microenvironment Modelling for Regenerative Medicine |
 |
| Funded by USYD-IPDF |
|
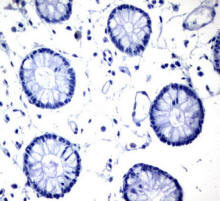
Understanding how tissue works in its microenvironment and how
changes in microenvironment regulate cell/tissue activities is
the key area and a question yet to be fully answered in
regenerative medicine. It is aimed in this project to establish
a theoretical framework of mechanical, chemical and biological
interactions between cell/tissue and their microenvironment.
|
| Modelling Tissue-Scaffold
System for Bone Regeneration |
 |
| Funded by ARC |
|
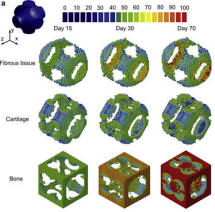
-
In collaboration with Prof. Qing Li (Sydney) and Prof. Juergen
Siepmann (Lille)
Biodegradable scaffolds play a critical role
in tissue engineering, in which the matrix degradation and
tissue ingrowth are of particular importance for determining the
ongoing performance of tissue-scaffold system during
regenerative process. This project focuses on understanding how
scaffold degradation and tissue regeneration compete with
each other during the bone regeneration process, where the
mechanical microenviroment is constantly changing thus
regulating the healing outcome. |
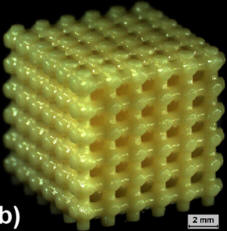
| Microstructural Design of
Biodegradable Porous Tissue Scaffold |
 |
| Funded by ARC |
| -
In collaboration with Prof. Qing Li (Sydney)
The goal of this research is to utilize topology optimisation as
a mathematical means for design and optimization of the tissue
scaffolds micro-architectures. To achieve this, a framework of
multi-objective topology optimization involving both mechanical
and fluidic criteria is developed, where effective stiffness of
scaffold is designed to match to host bone tissue while
the effective permeability is maximised under prescribed
porosity. For regulating bio-fluidic characteristics, a wall
shear stress uniformity criterion is also adopted to work with
non-gradient level-set method and bi-directional evolutionary
structural optimization method for achieving uniform wall shear
stress distribution on the scaffold microstructural surfaces and
a sustained lifetime of scaffold matrix subject to shear-induced
erosion. |
Yuhang Chen © 2012
|





